Abstract
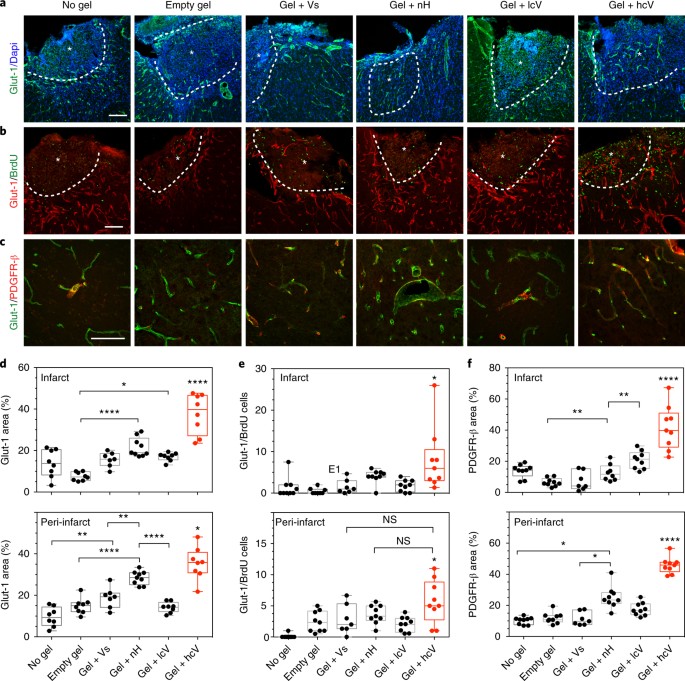
Stroke is the primary cause of disability due to the brain’s limited ability to regenerate damaged tissue. After stroke, an increased inflammatory and immune response coupled with severely limited angiogenesis and neuronal growth results in a stroke cavity devoid of normal brain tissue. In the adult, therapeutic angiogenic materials have been used to repair ischaemic tissues through the formation of vascular networks. However, whether a therapeutic angiogenic material can regenerate brain tissue and promote neural repair is poorly understood. Here we show that the delivery of an engineered immune-modulating angiogenic biomaterial directly to the stroke cavity promotes tissue formation de novo, and results in axonal networks along thee generated blood vessels. This regenerated tissue produces functional recovery through the established axonal networks. Thus, this biomaterials approach generates a vascularized network of regenerated functional neuronal connections within previously dead tissue and lays the groundwork for the use of angiogenic materials to repair other neurologically diseased tissue.
References
- 1.
Go, A. S. et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129, e28–e292 (2014).
- 2.
Larrivee, B., Freitas, C., Suchting, S., Brunet, I. & Eichmann, A. Guidance of vascular development: lessons from the nervous system. Circ. Res. 104, 428–441 (2009).
- 3.
Ohab, J. J., Fleming, S., Blesch, A. & Carmichael, S. T. A neurovascular niche for neurogenesis after stroke. J. Neurosci. 26, 13007–13016 (2006).
- 4.
Lindvall, O. & Kokaia, Z. Neurogenesis following stroke affecting the adult brain. Cold Spring Harb. Perspect. Biol. 7, a019034 (2015).
- 5.
Zhang, Z. G. et al. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J. Clin. Investig. 106, 829–838 (2000).
- 6.
Ergul, A., Alhusban, A. & Fagan, S. C. Angiogenesis: a harmonized target for recovery after stroke. Stroke 43, 2270–2274 (2012).
- 7.
Huang, L. et al. Glial scar formation occurs in the human brain after ischemic stroke. Int. J. Med. Sci. 11, 344–348 (2014).
- 8.
Sofroniew, M. V. & Vinters, H. V. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 (2010).
- 9.
Dirnagl, U., Iadecola, C. & Moskowitz, M. A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 22, 391–397 (1999).
- 10.
Fitch, M. T., Doller, C., Combs, C. K., Landreth, G. E. & Silver, J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 19, 8182–8198 (1999).
- 11.
Nih, L. R., Carmichael, S. T. & Segura, T. Hydrogels for brain repair after stroke: an emerging treatment option. Curr. Opin. Biotechnol. 40, 155–163 (2016).
- 12.
Nih, L. R. et al. Engineered HA hydrogel for stem cell transplantation in the brain: biocompatibility data using a design of experiment approach. Data Brief. 10, 202–209 (2017).